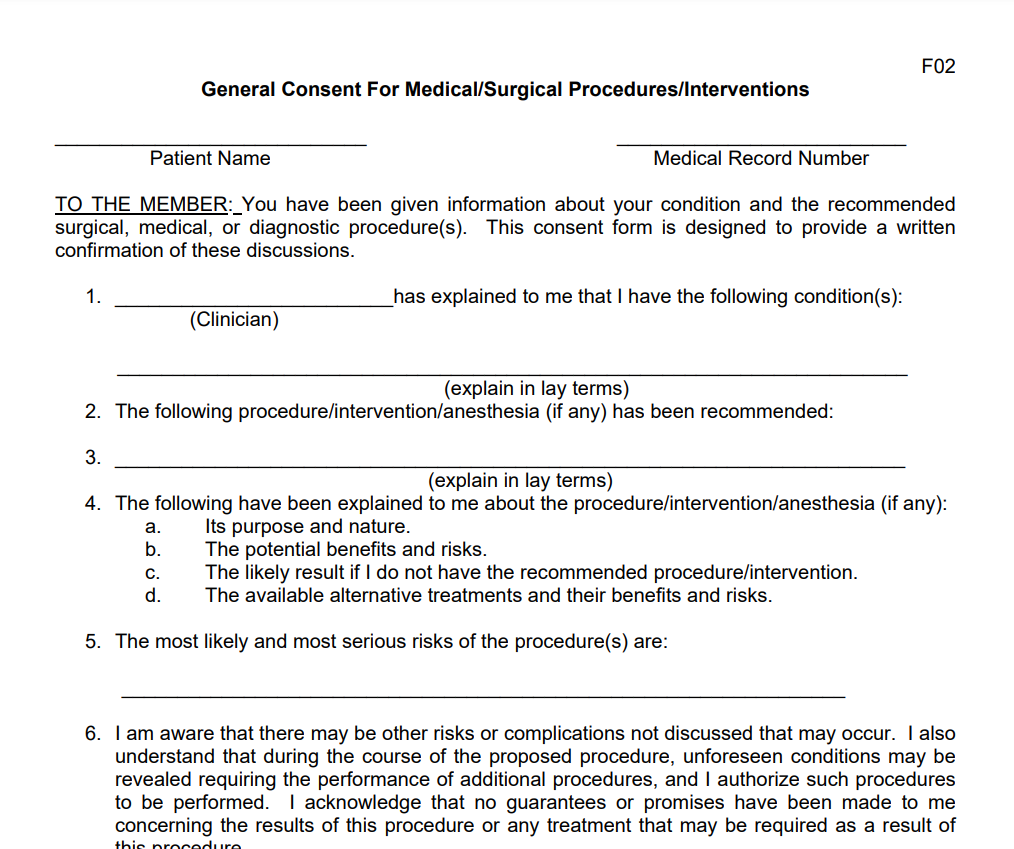
Procedure Consent Form – An Authorization for Procedure Form is a form of consent that informs a patient of what they can expect prior to undergoing an operation. It could be a straightforward document that asks for contact details for the patient as well as a signature and also the procedure’s risk and advantages. There are a variety of examples that you could use one of them to run your practice or business. Below, we’ve provided guidelines for creating an appropriate Procedure Consent Form to be used by medical facilities and doctors.
Informed consent
Informed consent is the method that allows a patient to understand the risks and advantages of a treatment prior to making a decision about whether or not to go through with it. It can be an overwhelming task. It also helps ensure patients have confidence that comes from knowing precisely what is going to happen throughout the procedure. Consent informed is an important component of the process of shared decision-making. It allows patients to make informed choices and make decisions regarding their health care.
The aim that informed medical consent has is to establish doctors and patients an effective team by describing the benefits and potential risks of treatment to both patients. Doctors should be aware of the concept of informed consent and the fundamental ethical guidelines that are the reason it is so crucial. In general, doctors must adhere to the principles of the law of informed consent for medical procedures that requires doctors to disclose all dangers and benefits of any procedure. Additionally, doctors must be aware of the possibility of liability if they perform procedures without consent from patients.
Consenting in a manner that is informed
Informed consent is a legally binding norm in the field of clinical medicine. Although it is mostly an ethical requirement, it also includes an important legal aspect. The consent form will show that you’ve spoken to the patient about the procedure and what it involves. The informed consent form may vary from institution to institution and can be documented in an oral form instead of a standard informed consent form. In any case informed consent should be recorded in the medical record of the patient.
A typical consent form could include a outline of the details about the procedure. A more detailed description may not be required, however the inclusion of a complete list makes the document more lengthy and difficult to comprehend. Summary of informed consent form as well as its revision, should provide the potential risks and benefits of the process. Informed consent form cannot guarantee complete confidentiality, but should instead should be based on FDA’s guidelines.
Informed consent forms
Consent informed forms for procedures are usually extremely limited in terms of the content. Just 13% of these documents include substantial details. They typically list that the process’s name without providing any specifics or description. While the literature on informed consent stresses the necessity of identifying the advantages and risks of procedures, many forms do not include them at all. Although most informed consent forms contain certain details, they may not be sufficient for educating the patient. The right time for discussions on informed consent is different for doctors and patients, and within the medical and family cultures. families.
Informed consent refers to the method where a patient freely opts to go through any procedure or treatment with the knowing and understanding the potential risks and benefits. It is an essential part of shared decision-making since it allows a patient to make an informed choice regarding their health. Following these guidelines informing consent, it can help patients make informed choices about their treatment. In this way, they’re more likely to be able to accept or decline treatments that they might not have otherwise selected.
Affirmative withdrawal of consent
The decision to revoke informed consent could be accomplished in a variety of ways. In the first instance, a patient could decide to sign a shorter form of consent form instead of a lengthy one. A shorter form that is an informed consent document could be an IRB-approved translation the lengthy form. A person may also fill out the English copy of the form. Patients can also choose to complete both forms in the event that it is a reflection of their awareness of the potential risks and benefits of this procedure.
The procedure that requires informed consent should be explained to the patient prior to the time the procedure is carried out. The language employed must be transparent and comprehensible without the use of words that are jargon-like or obscure. Additionally, it must not contain exculpatory language that is used to deny any legal rights of the participant. The language used is intended to relieve the sponsor, institution, or the investigator in the investigation from any liability when a participant is not willing to take part. Patients should seek IRB’s permission prior to granting the waiver of the written informed consent.
Download Procedure Consent Form 2024