Evoshield Consent Form – To comprehend the importance of an Evoshield Consent Form to understand the importance, we’ll first talk about the Authorized labeling manufacturing procedure, control strategy, and cold chain. Then, we’ll look at the impact of this form regarding the security and efficacy that the item provides. If you’re interested in getting the form, continue reading. This article covers these subjects and more. We’ll then look at the implications of this form for the patient. Evoshield Consent Form.
The government authorizes labeling.
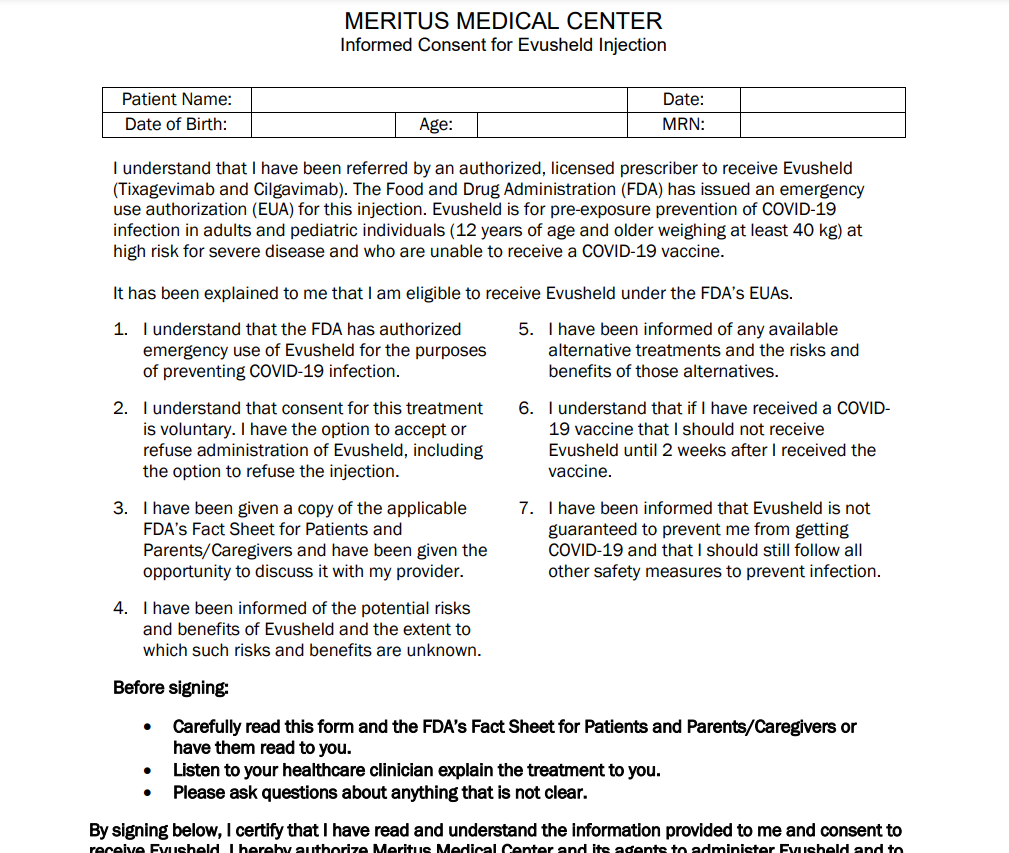
EVUSHELD should be administered alongside other vaccines, such as COVID-19, that must be administered at least two weeks following the administration of EVUSHELD. Every use of EVUSHELD should be documented in official Fact Sheets. AstraZeneca will make sure it is authorized labels distributed EVUSHELD. Furthermore, the consent form must contain the entire template Section headings.
The health facility has to maintain the inventory of its facilities that is accurate and records the use of the product. The facility should also keep documents about EVUSHELD storage and administration. The facility should also keep records that contain information about the patient, including the name, age, manifestations, and the number of doses given per patient. Additionally, the facility must keep its records up-to-date and supply them to FDA and AstraZeneca on demand.
Manufacturing process
To comply with EUA, AstraZeneca must maintain its inventory chain and ensure that it is reflected in that it uses EVUSHELD. In addition, the manufacturing process requires documents of the product administration and storage and information about patients, including names, age, date of birth, a manifestation of disease, and several doses given per patient. The manufacturer must keep such records in the event of EVUSHELD and any future modifications in the manufacturing procedure facilities, equipment, or control plan.
EVUSHELD must adhere to the requirements of the authorization letter. The letter of authorization includes the Fact Sheet and the Scope of Authorization. For patients with a weak immune system, the COVID-19 vaccination should be administered two months after EVUSHELD. Like any prescription drug, the use of EVUSHELD should be done by the approved Fact Sheet. AstraZeneca assures that the medication is distributed according to the correct markings.
Control strategy
The submission of new medicines is hampered because of a lack of coordination of strategies for controlling. Due to this, applicants must stagger when they submit new medications globally and must also respond to questions from global quality regulators. They typically submit one primary control strategy, but the same scientific principles are often interpreted differently according to different healthcare authorities. This can result in a significant level of variation in the number of questions asked. Additionally, applicants could be subject to many rounds of questions from a single regulator.
The distinction between methods of control for biological and synthetic substances is because certain countries have different processes for submission. This exists why there is a marked distinction in the methods used to control. Contrary to biologic drugs that are approved following the approval procedure, synthetic drug products have to be approved by various agencies. This means that the approval process for biological drugs should be simplified to make it easier for patients to access their therapies. Pharmaceutical companies should adopt ICH Q12 and manage their regulatory submissions to do this.
Cold chain and storage
An Evoshield Consent form to store or manage cold chains defines the need to keep vaccinations in good health. The document provides operational and regulatory specifications, packing and storage details, and four types of vaccines. The consent form includes any modifications to be made to the procedure after the product has been shipped. Beneath live a few of the numerous important points to be considered. If you’re an establishment that provides healthcare, you should ensure that all participants are aware of the conditions and terms. It is also necessary to offer the Authorized Fact Sheet to each patient caregiver and family member.
A Cold Chain Process: A vaccine should be stored at a temperature that is low throughout its storage and transport. The cold chain could vary from a normal 2-8 degrees Celsius to a complete freeze of at -70°F. The cold chain is a complex infrastructure and an extensive surveillance. This document from the CDC Vaccine Storage and Handling Toolkit highlights what is involved in this procedure. The process requires precise coordination among processes as well as temperature monitoring and accurate records for tracking. Regulations and other restrictions affect on the effectiveness of vaccinations.
Replacement requirements
In the case of a COVID-19-related pandemic the usage of EVUSHELD is only allowed under specific conditions. The consent form includes two vials for each individual. iRIS will change its number of the version number of the consent form as modifications are made to the data it includes. It is nevertheless necessary to be conscious that if the patient alters the information contained in their consent form, the patient must fill out an updated consent form.
Download Evoshield Consent Form 2024