Client Consent Form – How is a Client Consent Form obtained from a client? There are several ways to obtain a client’s consent. A client consent form, which you can access in “My Forms,” is the first approach. After completing the form, select “Send to Client” from the menu. The intake packet will then include the consent form. As an alternative, you might mail the form straight to the customer.
informed approval
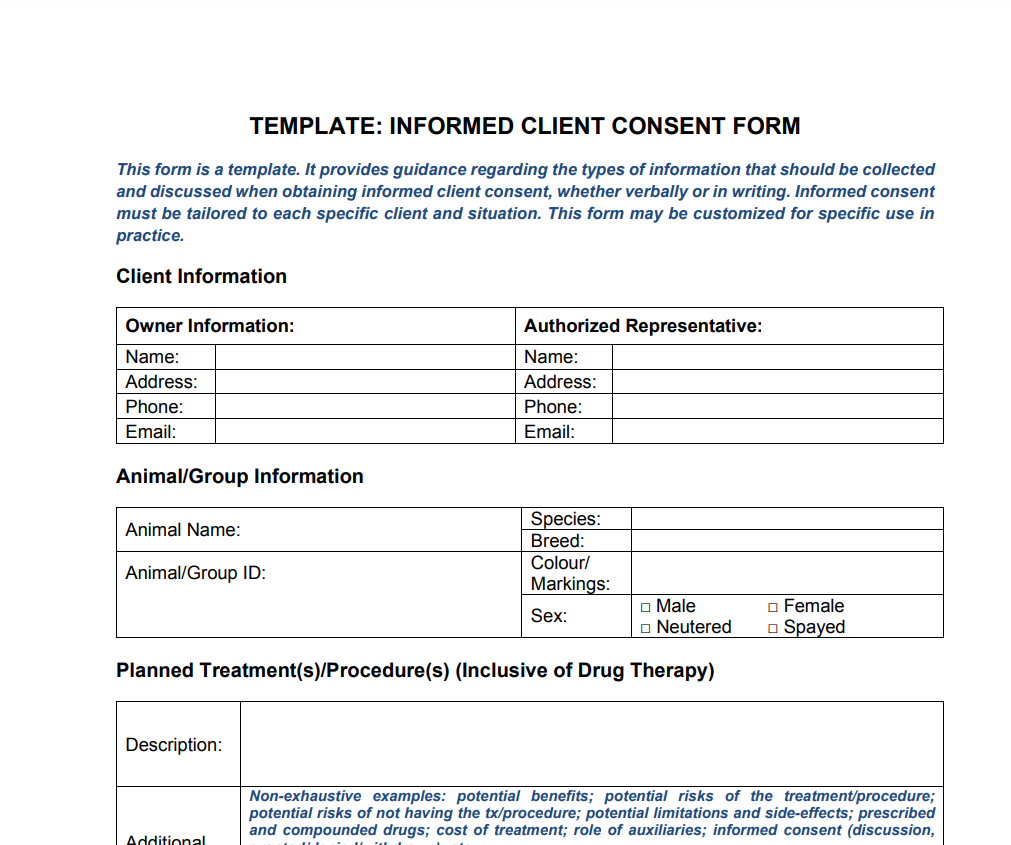
A patient must give consent before having medical procedures according to a legal document called an informed client consent form. Before beginning many complicated medical operations, informed consent is required. These include operations, chemotherapy, and cutting-edge diagnostic testing. Before receiving approval, the doctor must fully describe the advantages and disadvantages of a treatment. The informed client permission document is also admissible in court as proof. Even though it’s not usually the best option, sometimes a client consent form doesn’t need to be filled out.
Informed consent can be given in two ways: orally and in writing. Each of them has advantages and disadvantages, and each must be customized to the demands of the particular client. On an informed consent form, it should say what the procedure is, what the risks and benefits are, what options the patient has, and how well they understand items 1 through 4.
Explicit approval
You must obtain the express agreement of your clients before collecting and using their data for research. This could be implied by their actions or expressed verbally. Both forms of consent are required. Verbal express consent must be granted. Although the consent statement need not be in writing, it must be obvious that the subject accepts its requirements. You can give explicit consent by checking a “Yes” or “No” box or by making a statement.
According to GDPR, you cannot rely on a tick box for consent. Over time, consent typically deteriorates. This is particularly true if the consent is not granted promptly. The situation in which the consent is given will determine how long it lasts. For instance, a gym might want to send email newsletters to its customers with advice on good food or exercise over the summer. In this case, the user’s explicit permission would be needed for three different goals. However, the user’s newsletter subscription would end after the summer break.
Implied approval
The client’s legal right to consent to specific medical procedures is known as “implied consent.” A person’s actions, the truth, or their inactivity can all be used to infer this. For instance, the law presumes that a person has consented to medical care even if they may not have completely comprehended what was involved if they are unconscious during a car accident. Implied permission can be assumed when a patient is unconscious during treatment.
While implied consent is occasionally acceptable, it is not always acceptable. Some people are unable to give consent because they are mentally ill or are incarcerated. In certain circumstances, a parent or legal guardian must sign on the participant’s behalf. In other situations, participants’ assent may be sufficient to infer their permission. Implied consent is acceptable for research investigations and opinion polls where the subject is anonymous. It might not be good for marketing campaigns, though, because privacy laws require people to opt-in.
A research subject’s informed consent
Although the majority of informed consent studies have been on overseas research, the ethical concerns they raise do not apply to domestic research. Informed permission is crucial in both situations, even if the majority of domestic research uses a smaller pool of volunteers. In the subsequent area, we will examine at how knowledgeable consent can enhance domestic research. To do this, we will go over the fundamental ideas of informed consent and look at how they apply in various research scenarios. Knowing the most important parts of informed consent will help us make better decisions about how to do our research.
An interactive approach will also be a part of a successful informed consent procedure. Researchers should always take care to build rapport with potential study volunteers, even when face-to-face interactions may not be as beneficial as online ones. Key information can be explained using visual aids. Additionally, researchers might request that potential study volunteers describe key details in their own words while offering remedial comments and reassessing the situation as required. Participants will better comprehend the study as a result.
Consent to photography with knowledge
It’s common for a tourist to take a picture of a local without knowing exactly how or where the photo will be utilized. In addition, the tourist might not bother to inquire about whether the youngster or local would be willing to have their picture taken. A legal permission form might be a useful tool in this situation to ensure that the subject’s consent is obtained. It is important to get informed consent in order to protect both the photographer and the person being photographed.
A permission form should contain as much detail as feasible. What the photographer plans to do with the photos must be stated in detail. The photographer will be able to add more details with this method. It should also specify any restrictions that would make the participant reluctant to consent to the photos. Although the photographer may already be aware of the potential uses for the photos, it is still necessary to inform the subjects in advance. Electronic signatures on informed consent forms can be saved as digital files.
Download Client Consent Form 2024