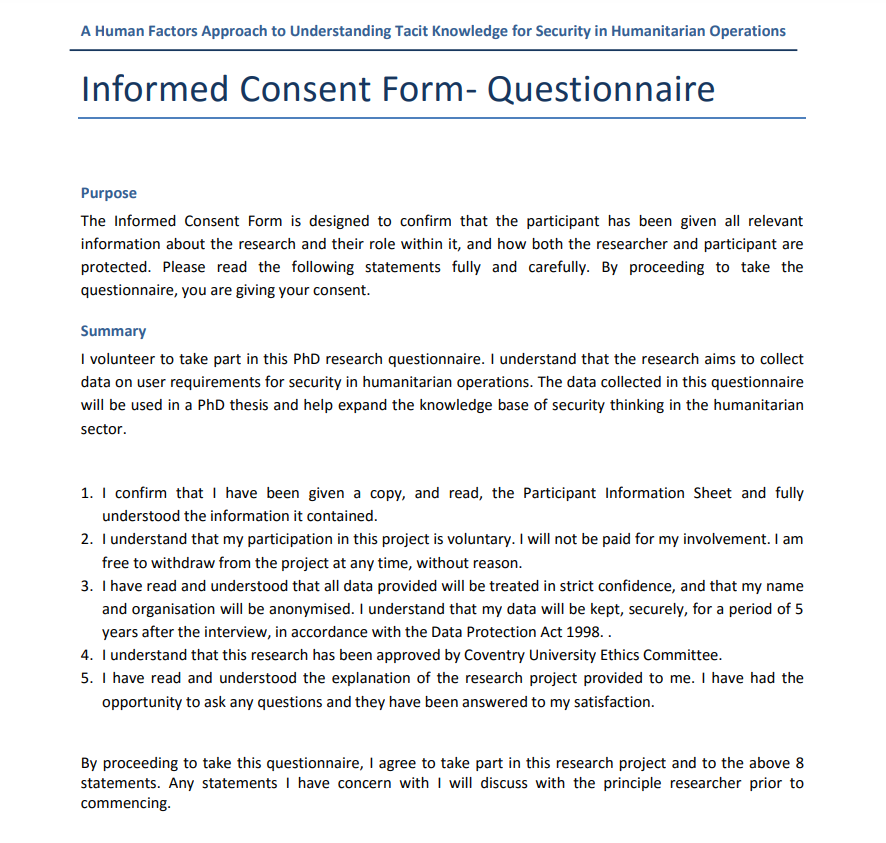
Consent Form For A Questionnaire – Human subjects research must be carried out in a way that enables potential subjects to make an informed choice. It is necessary to get informed consent under conditions that reduce the use of coercion or undue influence. Many subjects are restricted in their ability to make choices by circumstances. The participants’ freedom of choice may be limited by their employment, age, membership in particular groups, and physical and mental capabilities. Informed permission can also be obtained by using a questionnaire.
A record of the full consent process, an informed consent document is not a legal document. Information regarding the study and potential treatments for the subjects should be included. Subjects will have access to additional information, different kinds of consent, and services like medical or psychiatric counseling. A chance for them to ask any questions they may have will also be provided. These files will be included in a well-designed questionnaire. The procedure of obtaining informed consent ought to entail an education and consultation process with the subjects.
Text in the consent form
There exist a numeral of critical factors to take into account while constructing a questionnaire, beginning with the language. Both the researchers and the subjects should find it to be as straightforward and simple to understand as feasible. The consent form needs to be written in plain language that the subjects may easily understand. The questionnaire should be created for students in eighth grade, or at the very least, for the group you are researching. Do not use technical language; instead, compose the questionnaire as though you and the participants were having a discussion. It can be easier to clarify inclusion and exclusion criteria by using illustrations and bulleted lists.
The researchers might not understand the material if sophisticated terminology is used in consent forms. As a result, the permission form can be prepared at an unacceptable level. Using language suitable for grade six or eight is advised by the World Health Organization and the American Medical Association. The majority of consent forms, however, are written in complex language that is difficult for patients to grasp. Despite the fact that these suggestions are useful for researchers, they may make it difficult for patients to complete a questionnaire.
Conditions to get informed consent
By giving a brief explanation in a language that the potential subject may understand, informed permission can be obtained for a survey or questionnaire. The study’s potential participants ought to have enough time to consider whether or not they want to take part. They should also be made aware that participation is entirely optional and that their choice will not have any negative effects on the study. A questionnaire should provide details regarding the advantages and disadvantages of taking part in a research study in order to acquire informed consent.
Informed consent should guarantee that workers are completely informed about the study and that their decisions won’t be influenced in an improper way for one funded by their company. The consent form must emphasize that participation is voluntary as a doctor-patient connection may lead to a conflict of interest or negative outcome. Additionally, the PI must guarantee that participants may reach them at all times. Periodically, it can be required to remind participants of the research or its methods.
Formal format for consent
The permission form outlines the main points of the study as well as how to choose whether or not to participate. It should use plain language that non-academics may understand and should not use legal jargon or acronyms. The characteristics of the respondents’ demographics should also be taken into account. It should be clear to understand if there are plenty of young children, senior citizens, or people with special needs.
To preserve the confidentiality of the information submitted, it is crucial to adhere to rules when constructing a consent form. Construct infallible the font is big sufficiently for most people to read. Additionally, it needs to have a margin of at least 0.75″ on all pages, uniformly spaced. Informed written consent may not be required for surveys conducted over the phone or through Zoom. However, even if a consent form is not required, these forms nonetheless have to adhere to industry standards.
Download Consent Form For A Questionnaire 2024